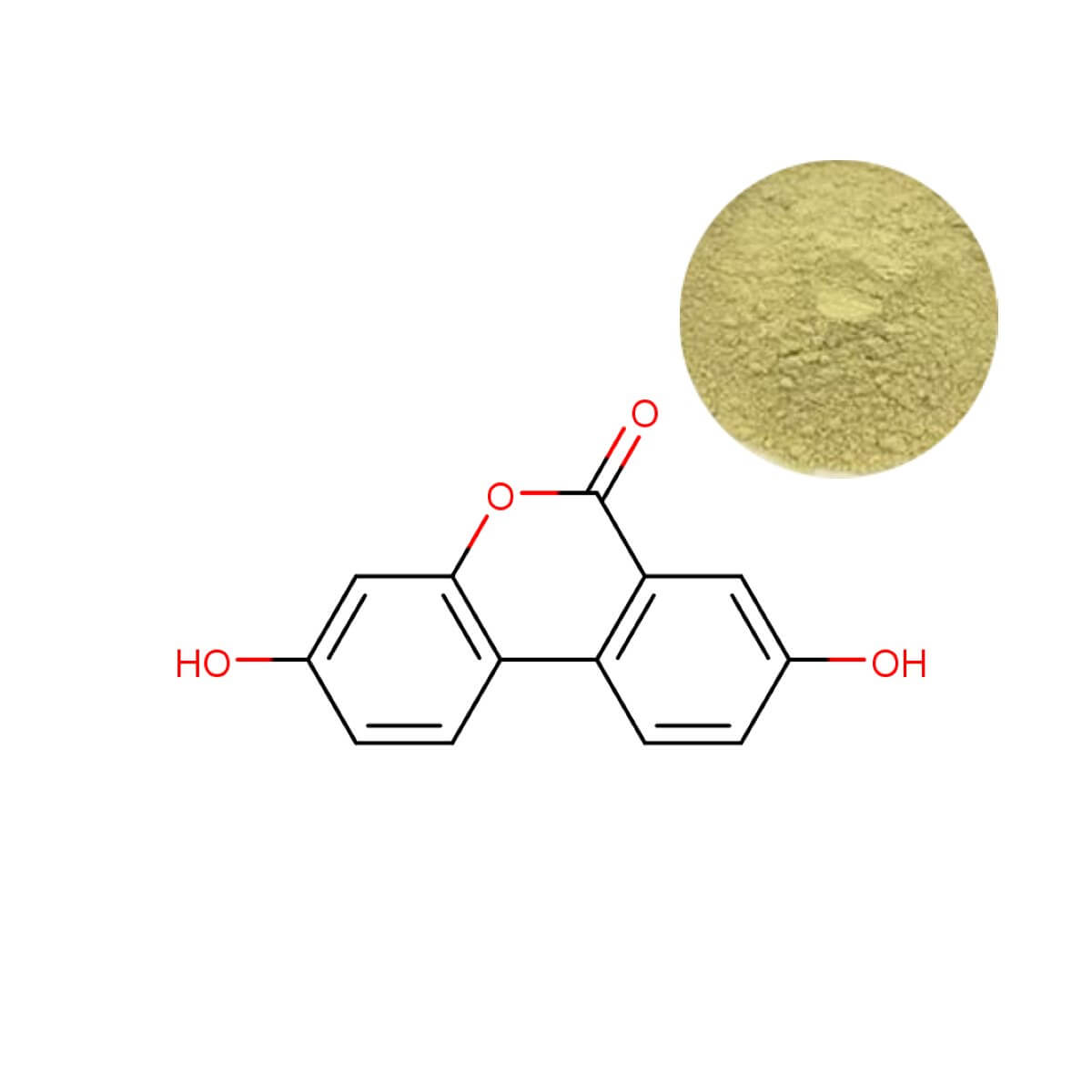
Urolithin A Powder
| Product Name | Urolithin A Powder |
| CAS Number | 1143-70-0 |
| Appearance | Grayish white to yellow fine powder |
| Purity | ≥98% (HPLC) |
| Packaging | 1 kg/bag, 5 kg/bag, 25 kg/drum |
| MOQ | 1 kg |
Urolithin A: The Strategic Ingredient for Differentiated Longevity Formulations
For R&D and procurement leaders, the challenge is no longer about adding another antioxidant to the list. It's about securing ingredients with a definitive mechanism to support both compelling consumer claims and rigorous regulatory scrutiny. Urolithin A (UA) answers this dual challenge. As a postbiotic with a direct role in cellular renewal, it offers a clear scientific narrative for brands targeting the sophisticated longevity market. However, its value is only realized when paired with uncompromising quality—where an HPLC-verified purity of ≥98.0% is the baseline, not the finish line. The following analysis provides a strategic framework for evaluating and integrating Urolithin A, focusing on the scientific, quality, and supply chain decisions that separate a market-leading product from a me-too formula.
The Postbiotic Mechanism: From Gut Metabolism to Cellular Housekeeping
Understanding the specific, science-driven story behind Urolithin A is the first step in building a defensible and marketable product. Unlike generic plant extracts, Urolithin A is a natural postbiotic metabolite produced by gut bacteria from dietary ellagitannins. Its primary role as a potent inducer of mitophagy—the cellular process of clearing dysfunctional mitochondria—addresses a fundamental aspect of aging at the cellular level. This is complemented by its anti-inflammatory properties, mediated through pathways like MAPK/PPAR-γ and Nrf2 activation (e.g., DOI: 10.1016/j.psj.2026.106448). For a formulation team, this precise mechanism provides the credible backbone for claims about supporting cellular energy and resilience, moving beyond vague wellness terminology.
The transition from mechanism to market is supported by targeted clinical research. A study in 54 obese adults showed that 1000 mg/day of Urolithin A significantly improved vascular endothelial function (measured by FMD) (NCT05921266). Another planned trial will examine its effects on glucose metabolism (NCT07353853). For a brand manager, this evolving clinical dossier is crucial. It means you can anchor your product’s positioning not just in cell biology, but in tangible human health outcomes related to metabolic and cardiovascular wellness—key concerns for your target demographic.
Decoding Quality: The Specifications That Dictate Market Success
In sourcing Urolithin A, the technical specifications on the CoA are not just a quality check; they are a direct determinant of your product’s regulatory viability and clinical efficacy. A strategic procurement approach evaluates these specs for both safety and functional performance.
| Quality Focus | Industry Benchmark | Strategic Procurement Implication |
|---|---|---|
| Assay Purity (HPLC) | ≥ 98.0% | Beyond ensuring potency, high and consistent purity minimizes batch variability, which is critical for replicating the dose-response seen in clinical studies. This consistency protects your product’s promised benefits. |
| Elemental Impurities | Meets USP FCC standards | Proactive compliance here prevents your finished product from failing independent lab testing for heavy metals—a common and costly point of failure for market entry. |
| Microbiological Controls | Conforms to USP ‹61›, ‹62› | This is a non-negotiable for GMP-aligned brands. It mitigates the risk of spoilage or recall, safeguarding your brand’s reputation in a category where trust is paramount. |
| Regulatory Status | Generally Recognized as Safe (GRAS) for intended use | Verifying GRAS documentation is a prerequisite for the U.S. market. It significantly de-risks your regulatory pathway compared to navigating the full NDI process with an unsubstantiated ingredient. |
Therefore, a meticulous quality audit extends beyond the CoA to process consistency. A parameter like Loss on Drying (LOD) consistently ≤0.5% is a telling indicator. For your manufacturing team, a low and stable LOD ensures predictable powder flow and mixing behavior, reducing production bottlenecks and ensuring each capsule contains the precise active amount. A supplier that controls this tightly likely has superior handling protocols from production to storage, reducing your hidden operational risks.
Formulation in Practice: Translating Science into Shelf-Stable Efficacy
The commercial success of a Urolithin A product hinges on the formulation team’s ability to overcome its physical challenges and unlock its biological potential. This is where practical expertise directly impacts consumer experience and repeat purchase.
Addressing the Bioavailability Challenge: Urolithin A’s poor aqueous solubility is the primary technical hurdle. The formulation strategy must be aligned with your product’s positioning:
- For Cost-Effective Capsules/Tablets: Standard direct blending is sufficient. A key operational insight is to pre-blend UA with a hydrophilic carrier (e.g., silica) before full-scale mixing. This simple step prevents clumping and ensures dose uniformity—a critical factor in achieving consistent clinical effects.
- For Premium or Liquid Formats: Here, the solubility challenge becomes a value-add opportunity. Sourcing a micronized form or a version using advanced delivery systems (e.g., phospholipid complexes) can enhance absorption. This allows for a stronger “advanced bioavailability” marketing story, justifying a premium price point.
Building a Synergistic Matrix: Urolithin A’s mechanism guides intelligent combination.
- With Nicotinamide Riboside (NR): This is a strategically sound pairing. While UA clears out damaged mitochondria, NR provides the NAD+ precursor to fuel new mitochondrial generation. For the consumer, this translates to a more comprehensive “cellular rejuvenation” story.
- Within a Broader Longevity Stack: Combining UA with other cellular health agents like fisetin can position a product at the cutting edge of the longevity category, appealing to informed consumers seeking multi-targeted solutions.
Mitigating Supply Risk: The Due Diligence Framework for Strategic Sourcing
For a novel, clinically-backed ingredient, supply chain decisions have long-term strategic consequences. A thorough due diligence process focuses on documentation and capability, transforming sourcing from a transactional cost center into a competitive advantage.
The Documentation Audit: This is your first line of defense.
- Batch-Specific CoA: Treat this as the ingredient’s passport. Verify every parameter, especially the CAS number (1143-70-0) and assay method. Inconsistency here is a red flag for broader quality issues.
- GRAS Evidence: Do not accept a marketing claim. Request the supporting documentation, such as the expert panel conclusion or FDA correspondence. This step alone can prevent a year-long regulatory setback.
- Stability Support: A supplier invested in the ingredient’s success will provide data to justify storage claims and shelf life, reducing your product stability validation burden.
The Supplier Capability Assessment: This evaluates future-proofing.
- GMP Certification: This is a baseline requirement for any serious brand. It assures that the material is produced under a quality management system aligned with global standards.
- Scale and Contingency Planning: Inquire about capacity and backup plans for raw materials. For a brand planning a major launch, a supplier’s inability to scale or its reliance on a single-source input represents a significant business risk.
- Technical Partnership Potential: The most valuable suppliers act as extensions of your R&D team. Can they provide application data, co-develop custom blends, or troubleshoot formulation issues? This level of support is a key differentiator for brands aiming to innovate rather than imitate.
From a Total Cost of Ownership (TCO) perspective, selecting a partner based on this comprehensive framework often leads to a lower true cost. The initial price per kilogram is offset by avoiding the immense costs of regulatory delays, product rework, supply disruption, or—worst of all—a failed market launch due to inconsistent quality.
Validating the Strategic Fit for Your Brand
Urolithin A offers a clear pathway to a differentiated position in the high-growth longevity segment. The decision to proceed should be informed by hands-on validation that aligns with the strategic considerations outlined above. A phased approach to de-risk integration.
Request a complimentary sample batch alongside the complete technical dossier. This allows your team to conduct in-house verification testing, assess organoleptic properties, and conduct a pilot production run. Critically, reviewing the full dossier—including GRAS documentation and detailed specifications—enables a thorough compliance and quality audit before any commitment is made. This step transforms a sourcing decision from a speculative gamble into a data-driven strategy. Request Your Complimentary Sample & Technical Dossier
Frequently Asked Questions
Share this product
Related Products
Ready to get started?
Contact our team for technical specifications, pricing, and customized solutions.