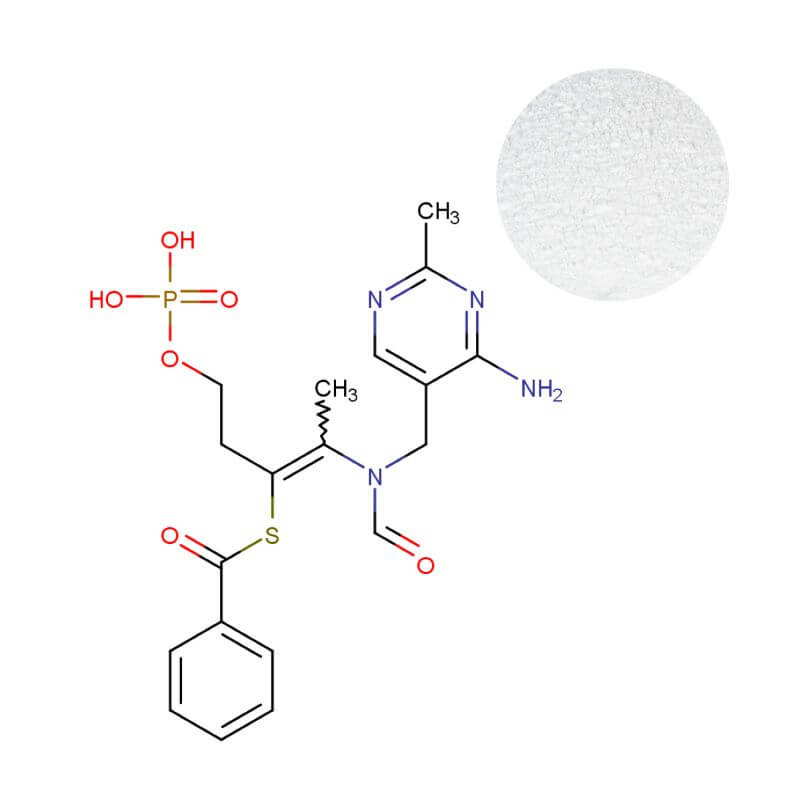
Benfotiamine Powder
| Product Name | Benfotiamine Powder |
| CAS Number | 22457-89-2 |
| Appearance | White to off-white solid powder |
| Purity | ≥98% (HPLC) |
| Packaging | 1 kg/bag, 5 kg/bag, 25 kg/drum |
| MOQ | 1 kg |
Maximizing Thiamine Efficacy: Why Forward-Thinking Formulators Choose Benfotiamine
When your clients demand supplements with discernible results, relying on ordinary thiamine is a strategic gap. In today's competitive market, where ingredient transparency and efficacy drive purchasing decisions, formulators need a definitive edge. Benfotiamine provides that edge, not merely as a thiamine source, but as a bioavailability-engineered solution that translates directly to superior product performance and consumer loyalty. This is why it’s rapidly moving from a niche ingredient to a mainstream staple in premium formulations.
Consider this: with the global market for specialized nutraceuticals expanding, products must deliver on their promises from the first bottle. Benfotiamine's proven absorption advantage solves the core challenge of converting a raw material into a tangible consumer experience, making it one of the highest-ROI upgrades for your formula.
What Makes Benfotiamine Different (And Why This Difference Builds Your Brand)
Ordinary thiamine salts like the common Vitamin B1 Thiamine HCl face a fundamental pharmacokinetic barrier: their water-solubility limits efficient passage through lipid-rich cell membranes. Think of it as trying to move through a system designed for fat while you’re covered in water.
Benfotiamine’s fat-soluble, synthetic structure—S-benzoylthiamine O-monophosphate—acts as a biochemical passport. It bypasses the saturation-limited, active transport channels that restrict standard B1, utilizing passive diffusion for rapid and efficient uptake. The outcome isn't marginal; peer-reviewed studies consistently show blood thiamine levels 3-5 times higher than with equivalent doses of thiamine hydrochloride.
But here’s the critical takeaway for you as a formulator: this pharmacokinetic advantage isn't just a lab statistic. It directly fuels the functional efficacy that builds brand reputation. When your nerve support or metabolic health formula delivers noticeable benefits, you're not just selling a supplement; you're securing a loyal customer. This direct link from superior science to consumer-perceivable results is where your premium positioning is validated.
Where Smart Formulators Are Deploying Benfotiamine for Market Advantage
The application landscape for benfotiamine is expanding with robust clinical backing. Here’s how leading brands are integrating it to create defensible market positions:
- Next-Generation B-Complex & Multivitamins - Differentiation in crowded markets is key. Featuring benfotiamine as your thiamine source creates a tangible, science-backed absorption story that elevates your entire formula from a commodity to a performance-driven blend.
- Targeted Neurological & Nerve Health Formulas - This is where the research is most compelling. A 2022 review in Diabetes Research and Clinical Practice confirmed its effectiveness and excellent safety profile in managing symptomatic diabetic neuropathy. Formulators use it as a cornerstone for products aimed at neurological comfort, maintenance, and antioxidant support for nervous tissue.
- Advanced Metabolic Health Systems - Beyond basic energy conversion, benfotiamine supports healthy glucose metabolism pathways by activating transketolase. This makes it indispensable in sophisticated, multi-mechanism formulas designed for comprehensive metabolic care, a sector growing in double digits.
- Comprehensive Antioxidant & Cellular Protection Blends - Often underestimated, benfotiamine exhibits direct, non-coenzyme antioxidant properties. For holistic wellness products, this dual action—both as a essential vitamin and a cell protector—delivers exceptional formulation value.
- Performance-Driven Sports Nutrition - Athletes invest in outcomes. The guaranteed higher systemic availability means the active compound reaches muscle and nerve tissues more efficiently, supporting energy metabolism and recovery under physical stress—benefits that active consumers can truly feel.
The Quality Discussion That Protects Your Brand (And Your Bottom Line)
In B2B sourcing, not all benfotiamine is created equal. The variance lies in a quality assurance protocol that views specifications as a starting point, not the finish line. Your brand’s integrity depends on the consistency and purity of your raw materials.
When we partner with you, our evaluation focuses on the parameters that prevent manufacturing headaches and safeguard your market entry:
- HPLC-verified purity consistently ≥98% – backed by Certificates of Analysis for every batch, not just occasional audits.
- Microbiological controls exceeding standard limits – because contamination can halt your production line and jeopardize shelf-life.
- Rigorous heavy metal screening – adhering to the strictest global standards (USP, EP) to ensure your product passes independent lab testing.
- Real-world stability and compatibility data – providing confidence that the ingredient performs not just at arrival, but throughout your product's entire lifecycle.
Our commitment: We provide full, batch-specific documentation within 24 hours of your request. This transparency isn't just paperwork; it's your insurance policy for seamless production and unwavering consumer trust.
Managing Your Supply Chain for Predictable Growth
The past years have taught every formulator that a brilliant product is useless if you can’t source its key ingredients. Reliability is now as critical as efficacy.
We mitigate supply chain risk through a strategic approach designed for your long-term success:
- Multi-source manufacturing partnerships to prevent single-point failures and ensure volume flexibility.
- Demand-driven inventory planning that accounts for true lead times, including production, QA, and logistics.
- Pre-validated regulatory compliance for major markets (US DSHEA, EU Novel Food, ANVISA, etc.), simplifying your global expansion.
- Proactive communication on market dynamics – we believe in forecasting challenges, not just reacting to them.
The goal is simple: to be the partner who ensures that the benfotiamine you qualify today remains available, consistent, and compliant two years from now when your brand is a category leader.
Practical Technical Integration for Flawless Manufacturing
Let’s translate science into production reality. How does benfotiamine behave on your manufacturing floor?
Based on aggregated technical support data from hundreds of production runs:
Tableting: It compresses well but achieves optimal hardness and stability with specific excipient combinations. We advise on filler-binder systems that protect integrity during compression and coating.
Capsules (Two-piece & Veggie): The powder demonstrates excellent flowability at standard manufacturing parameters. The key technical note: maintain processing area relative humidity below 45% during encapsulation to ensure optimal powder characteristics.
Powder Blends & Stick Packs: It mixes uniformly but requires particle size matching to your base powder to prevent segregation. We provide guidance on optimal mesh sizing (typically 80-100 mesh) for different blend compositions.
The bottom line for your operations: This understanding helps you avoid costly trial-and-error, optimize run times, and guarantee that the bioavailability advantage in the lab translates perfectly into every finished unit you produce.
Making the Strategic Switch: Key Formulator Considerations
Transitioning from ordinary thiamine to benfotiamine is straightforward with the right partner. Here’s what to plan for:
- Precise Dosage Equivalency: Due to its 3-5x greater bioavailability, you achieve equivalent or superior thiamine activity with a lower mass of benfotiamine. The exact ratio depends on your baseline (whether you're switching from Thiamine Mononitrate or HCI) and your target efficacy level.
- Proven Stability: Benfotiamine maintains excellent potency under standard, climate-controlled storage conditions. We provide validated stability data to inform your packaging and shelf-life decisions.
- Claim & Compliance Strategy: Regulatory pathways differ by region. We support you with the necessary dossiers, structure/function claim guidance, and compliance documentation (FSSC, cGMP) to accelerate your market entry.
This transition typically offers one of the clearest returns on investment in formulation. While the raw material cost is higher than basic thiamine, the performance premium—justified by clinical data and consumer outcomes—delivers a multiplier effect on your brand's value perception.
Ready to Elevate Your Formulas with Confidence?
Choosing benfotiamine is a decision that resonates from your lab to your end-consumer. It represents a tangible upgrade in product integrity and market potential.
To summarize, partnering with us for your benfotiamine supply means:
① Accessing a clinically-validated, high-performance ingredient with a definitive bioavailability story.
② Reducing compliance risk and time-to-market with pre-assembled regulatory and technical documentation.
③ Securing a reliable, transparent supply chain designed to support your growth, not just fulfill an order.
Frequently Asked Questions
Share this product
Related Products
Ready to get started?
Contact our team for technical specifications, pricing, and customized solutions.