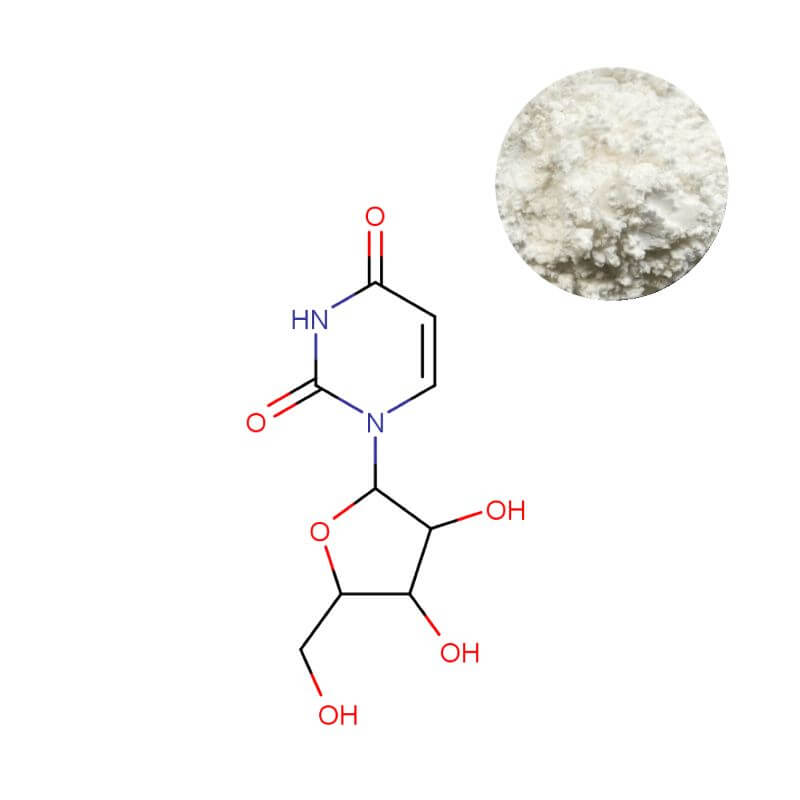
Uridine Powder
| Product Name | Uridine Powder |
| CAS Number | 58-96-8 |
| Appearance | White to off-white crystalline powder |
| Purity | ≥99% (by HPLC) |
| Packaging | 1 kg/bag, 5 kg/bag, 25 kg/drum |
| MOQ | 1 kg |
Uridine: The Foundational Nucleotide for Advanced Cognitive Health Formulations
For brands navigating the saturated cognitive health market, the pressing challenge is no longer just adding ingredients, but integrating those with a definitive, mechanistically clear role in brain physiology. This shift moves the focus from short-term stimulation to long-term structural support, demanding raw materials that offer both scientific substantiation and formulation versatility. Uridine, an essential pyrimidine nucleoside, meets this demand by serving as a direct nutritional precursor for the synthesis of neuronal membrane phospholipids, with emerging research highlighting its role in systemic metabolic regulation (DOI: 10.3389/fnut.2025.1651993). Sourcing premium uridine powder, characterized by ≥99% HPLC-verified purity and definitive identity markers like a specific rotation of +8° to +10°, is a critical first step in building a credible nootropic product. The material’s consistency, driven by stringent quality benchmarks, directly determines the efficacy and reliability of the finished formulation. This analysis provides a strategic framework for product developers to leverage uridine's unique science, application-specific handling, and quality determinants to create differentiated and clinically-substantiated products.
The Synaptic Connection: Uridine as a Rate-Limiting Precursor for Phospholipid Synthesis
Understanding uridine's core mechanism is key to formulating products that credibly support long-term cognitive function, rather than offering transient effects. Its primary value lies in its foundational role: upon ingestion, uridine is phosphorylated to uridine triphosphate (UTP), a direct precursor for synthesizing cytidine diphosphate-choline (CDP-choline). This positions uridine as an obligatory nutritional component for producing phosphatidylcholine, the most abundant phospholipid in neuronal and synaptic membranes. Crucially, this pathway is most efficacious when uridine is co-administered with the omega-3 fatty acid DHA and a choline source (e.g., Alpha-GPC), forming a synergistic "triad" that collectively accelerates the construction of synaptic infrastructure. This mechanism is directly supported by clinical evidence; a 2023 pilot study administering a complex containing uridine, phosphatidylserine, and choline to older adults with mild cognitive impairment demonstrated significant improvements in standardized cognitive scores (MMSE/MoCA-Ina) and favorable shifts in quantitative EEG patterns (DOI: 10.30574/wjarr.2023.19.2.1582). For a brand, this mechanistic clarity translates into a powerful product narrative. It allows claims to pivot from generic "brain boosting" to scientifically-grounded "support for memory and learning functions," by directly nourishing the brain's synaptic plasticity. This depth of science is particularly valuable for brands targeting informed consumers or entering clinical-facing wellness channels, where mechanistic plausibility is a key purchasing driver.
Decoding Quality: Why Purity Assays and Specific Identity Tests Are Non-Negotiable
In B2B sourcing, the technical specification sheet is a risk-mitigation document. For a molecule like uridine, where efficacy depends on its specific biochemical form, identity tests are as critical as purity assays for ensuring you receive a functionally active material. A ≥99% HPLC assay is a necessary baseline, but it does not guarantee the correct stereochemistry or the absence of isomeric impurities that lack biological activity.
| Quality Parameter | Specification & Typical Value | Commercial & Functional Rationale |
|---|---|---|
| Specific Rotation [α]²⁰/D | +8.0° to +10.0° (c=2, in H₂O) | This definitive identity test confirms the correct D-ribose isomer of uridine. A value outside this range signals impurities, an incorrect form, or degradation, directly compromising the material's utility in the phosphatidylcholine synthesis pathway and rendering it ineffective for its intended purpose. |
| Melting Point | 165°C to 168°C | This classic purity indicator signifies a highly pure, crystalline compound. A broad or depressed range suggests contaminants, which can introduce batch-to-batch variability in manufacturing. |
Therefore, a comprehensive Certificate of Analysis (CoA) must seamlessly integrate potency with identity. For elemental impurities, alignment with modern standards like USP <232> and <233> via ICP-MS is essential. This demonstrates a supplier's commitment to current pharmacopoeial compliance, going beyond basic heavy metals screening. From a procurement perspective, this dual focus materially de-risks the supply chain. It provides auditable proof that the raw material is both potent and authentic, which is fundamental for defending your own product's quality claims during a regulatory audit or quality assurance review. Investing in a supply partner that provides this level of analytical detail is a proactive measure to prevent costly formulation failures or compliance issues downstream.
Formulation in Practice: Building Synergy, Ensuring Stability, and Avoiding Pitfalls
The transition from a quality raw material to a successful finished product hinges on practical formulation intelligence. For uridine, this means strategically leveraging its synergies and meticulously managing its physical properties. Commercially, uridine's highest value is realized in synergistic stacks, not as a standalone ingredient. The most evidence-backed combination pairs uridine (in a typical clinical range of 250-500 mg/day) with a bioavailable choline source (like Alpha-GPC or Citicoline CDP Choline Powder) and an Omega-3 complex rich in DHA. This trio directly supplies the three substrates required for the Kennedy pathway, making the formulation's support of phospholipid synthesis both efficient and clinically plausible.
However, the most common technical failure point is overlooking uridine's hygroscopic nature. This physical property dictates critical handling protocols that directly impact manufacturing efficiency and shelf-life:
- In Manufacturing: Processing and blending should occur in a controlled environment with low relative humidity (<45%). This practical prerequisite is often overlooked yet more critical for product consistency than simply specifying a high-purity grade. Failure to control humidity can lead to caking and poor powder flow, causing inaccurate dosing and production line delays.
- In Packaging: For bulk material, double-barrier packaging with an inner foil bag is standard. For the finished product, high moisture-barrier packaging is non-negotiable. Brands should engage packaging suppliers early to ensure the chosen material's water vapor transmission rate (WVTR) is appropriate for a hygroscopic formulation, protecting the active ingredients throughout the product's declared shelf life.
Mitigating Supply Risk: A Checklist for Sourcing Strategic-Grade Uridine
Selecting a uridine supplier is a strategic decision that impacts long-term product consistency and supply chain resilience. Experienced procurement teams evaluate beyond price-per-kilo, assessing partners on technical capabilities and quality system maturity. The following framework serves as an objective due-diligence checklist to mitigate supply risk:
- Documentation & Transparency: Does every batch come with a comprehensive CoA that includes both potency (HPLC assay) and full identity confirmation (Specific Rotation, Melting Point)? Is the MSDS detailed and readily available? Consistent documentation is the first indicator of a disciplined quality system.
- Methodology & Regulatory Alignment: Are analytical methods for impurities (e.g., heavy metals via ICP-MS) aligned with current standards like USP <233>? Does the supplier's quality framework support compliance with dietary supplement GMPs (21 CFR Part 111)? This is crucial for brands selling in regulated markets like the US and EU.
- Supply Chain Proof Points: Can the supplier demonstrate a reliable supply track record and provide industry-standard, moisture-resistant packaging options (e.g., 1kg, 5kg, 25kg)? A history of supply consistency often outweighs a marginal cost saving.
- Technical Partnership Capacity: Can the partner provide application support, such as stability data or formulation guidance? This transforms a vendor into a solutions partner, reducing development time and technical risk.
For a non-branded, synthetically-produced ingredient like uridine, this due diligence focuses overwhelmingly on analytical rigor, regulatory savvy, and process consistency. The goal is to secure a supply that acts as a silent, reliable foundation—never becoming a variable in your finished product's performance or a vulnerability in your supply chain.
Next Steps for Your Cognitive Health Product Development
Integrating uridine into a cognitive health formulation represents a strategic move towards products supported by foundational neuroscience. Its role in synaptic membrane synthesis offers a credible and differentiating story for brands aiming to transcend the standard nootropic landscape. The most effective way to evaluate its fit for your specific product line is through practical, hands-on assessment. To support this critical phase, we recommend requesting your complimentary sample & technical dossier. This dossier provides the detailed CoA exemplars, handling protocols, and application insights needed to inform your development timeline, assess manufacturability, and de-risk your path to a successful product launch.
Frequently Asked Questions
Share this product
Related Products
Ready to get started?
Contact our team for technical specifications, pricing, and customized solutions.