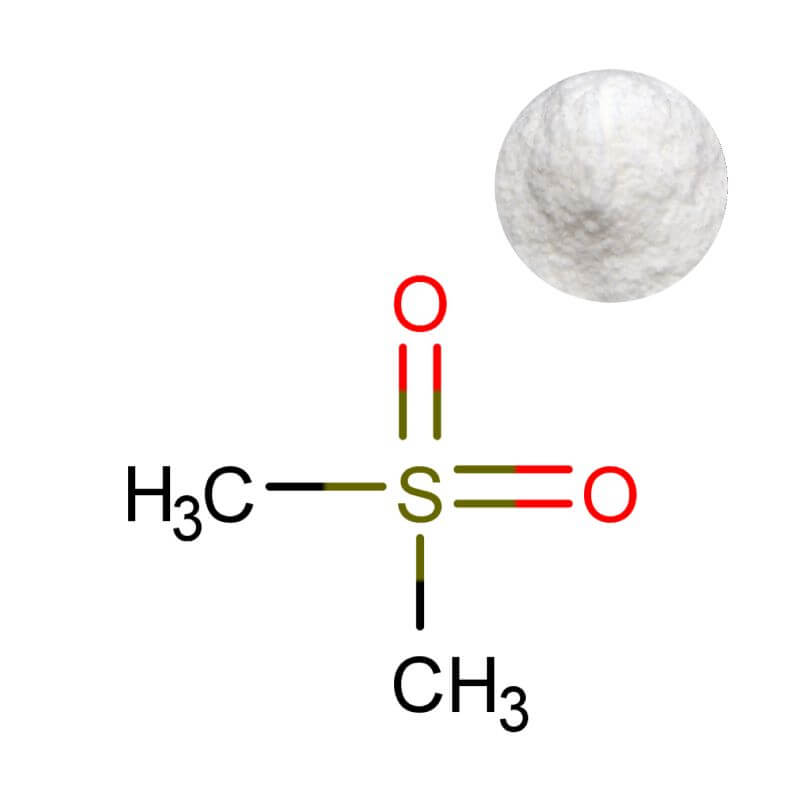
MSM Powder
| Product Name | MSM Powder (Methylsulfonylmethane / Dimethyl Sulfone) |
| CAS Number | 67-71-0 |
| Appearance | White crystalline powder |
| Purity | ≥99% (by HPLC) |
| Packaging | 25 kg/bag |
| MOQ | 25 kg |
MSM Powder: Clinically-Supported Joint and Muscle Support for Sports Nutrition Formulations
In the competitive sports and active nutrition market, product differentiation now critically depends on clinically-substantiated ingredients and supply chain integrity. For brands, incorporating Methylsulfonylmethane (MSM) is a strategic move to meet evidence-based consumer demands for recovery and joint health. The commercial return on this investment, however, is determined by the verifiable quality of the raw material and the depth of technical support behind it. Sourcing premium MSM powder, standardized to a minimum of 99.0% purity with robust clinical backing, is essential for building a credible and efficacious formula. This guide provides a decision-making framework for R&D and procurement teams, translating scientific data and quality specifications into actionable insights for product development and supplier selection.
The Dual-Action Mechanism: How MSM Supports Connective Tissue and Modulates Exercise-Induced Stress
For formulation scientists, understanding MSM's dual mechanism is key to designing effective products and crafting compelling, compliant messaging. This knowledge directly informs dosage decisions and target audience selection.
Fundamentally, MSM is a bioavailable source of organic sulfur, a critical element that constitutes approximately 34% of the body's connective tissues like cartilage, tendons, and ligaments. This structural role is fundamental for joint integrity. Beyond this, modern research reveals a significant secondary function: MSM exerts a modulating effect on the body's inflammatory and oxidative stress pathways triggered by intense physical activity. This dual action—providing foundational building blocks and helping to manage the biochemical stress of exercise—explains its broad application from general joint health formulas to targeted sports recovery blends.
A critical commercial takeaway is the well-established dose dependency of clinical efficacy. For instance, a 2023 systematic review concluded that supplementation with 3 to 5 grams per day for at least two weeks is effective in reducing markers of exercise-induced muscle damage and oxidative stress (doi: 10.3390/nu15132995). For a brand manager, this defines the minimum effective dose required to deliver the consumer-perceivable benefits of faster recovery and reduced soreness that drive product repurchase. Formulating below this range risks a clinically-ineffective product, while adhering to it provides a defensible foundation for structure/function claims and justifies a premium market position.
Beyond Purity: Decoding the CoA for True Quality and Stability Assurance
Procurement teams understand that a purity claim is just the starting point. A deep dive into the Certificate of Analysis (CoA) reveals the factors that guarantee in-production performance and finished product shelf-life, directly impacting operational efficiency and brand safety.
| Critical Parameter | Expert Benchmark | Commercial Implication |
|---|---|---|
| Loss on Drying (Moisture Content) | ≤ 0.5% | MSM is hygroscopic. Low moisture is critical for maintaining free-flowing powder properties, preventing caking during storage, and ensuring consistent blending in your production line. A specification exceeding this benchmark can lead to manufacturing delays, batch inconsistencies, and increased waste—hidden costs that erode initial savings from a cheaper supplier. |
| Heavy Metals & Residues | Meeting stringent limits for Lead, Arsenic, Cadmium, and Mercury | This is a fundamental compliance and brand protection checkpoint. Insisting on full ICP-MS testing from a supplier, rather than basic screening, mitigates downstream regulatory risk and protects against consumer safety issues that can irrevocably damage brand equity. |
Furthermore, forward-thinking brands can leverage emerging research for advanced storytelling. A 2024 animal model study demonstrated that MSM supplementation helped preserve joint structure in post-traumatic osteoarthritis, suggesting potential chondroprotective properties (doi: 10.1002/jor.25795). For product managers, this mechanistic research supports a more sophisticated market positioning. It allows the brand narrative to evolve from simply "managing discomfort" to "supporting long-term joint health and active mobility," a compelling message for an aging yet active consumer demographic.
Formulation in Practice: Synergy, Delivery, and Avoiding Common Pitfalls
Moving from ingredient selection to successful product launch requires practical application knowledge. Strategic formulation choices are what transform a standard ingredient into a differentiated, high-performance product.
Strategic Synergistic Combinations for Market Differentiation:
- With Glucosamine and Chondroitin Sulfate: This classic combination is a market expectation for joint health. Formulating this trio addresses multiple biochemical pathways, creating a comprehensive solution that is difficult for competitors to undermine with a single-ingredient alternative. It justifies a higher price point and appeals to consumers seeking complete support.
- With Sodium Ascorbate and Hyaluronic Acid (Sodium Hyaluronate Powder): This combination enables premium, multi-benefit positioning for both joint and skin health. Since Vitamin C is essential for collagen synthesis—a sulfur-dependent process—pairing it with MSM creates a scientifically coherent story for "beauty from within" or holistic wellness products.
Application-Specific Insights to De-Risk Production:
- Capsules & Tablets: The specified particle size (≥95% through 80 mesh) is critical for operational efficiency. Consistent flow ensures uniform die filling, guaranteeing content uniformity in every unit. A coarser, inconsistent powder can lead to dosage inaccuracies, failed QC checks, and product recall risks—costly outcomes that far outweigh any minor savings on the raw material.
- Powdered Drinks & Stick Packs: While MSM's high solubility is an asset, managing its hygroscopicity during production is key. Controlling the blending environment humidity (<45% RH) is a practical, often-overlooked step that does more to ensure long-term stability and prevent clumping than packaging alone. This operational insight can be the difference between a product that remains free-flowing for its entire shelf life and one that develops consumer complaints.
Mitigating Supply Chain Risk: A Checklist for Sourcing Premium MSM
In a commoditized market, the true cost of an ingredient encompasses far more than its price per kilo. A rigorous supplier evaluation framework protects your brand from hidden costs related to quality failures, regulatory delays, and supply disruption.
When evaluating a bulk MSM supplier, experienced procurement and quality teams treat the following as non-negotiable criteria:
- Documentation Depth & Transparency: Insist on a comprehensive, batch-specific CoA detailing every parameter from purity and moisture to full heavy metals and microbiology panels, with test methods (e.g., USP, HPLC) clearly stated. This document is your primary quality guarantee.
- Regulatory Preparedness and Support: The supplier should proactively provide documentation supporting use in your target markets. For the US, this includes understanding its GRAS status; for the EU, clear guidance on its status as a permitted food ingredient. Their ability to support your regulatory dossiers marks them as a true partner.
- Proof of Process Control and Consistency: Request CoAs from three consecutive production batches. Analyze the variance in key specs like purity and moisture. Minimal fluctuation (e.g., ±0.2%) is a direct indicator of a controlled, GMP-certified manufacturing process and is the single best predictor of future batch reliability.
- Value-Added Technical Partnership: Assess whether the supplier offers formulation guidance, stability data, or regulatory overviews. This shift from a transactional vendor to a solutions partner provides invaluable R&D support and can accelerate your time-to-market.
Applying this framework systematically allows you to compare suppliers on objective risk factors, not just price. The goal is to select a partner whose operational excellence and documentation rigor directly contribute to the resilience and quality of your own finished product line.
Next Steps for Your Product Development
Strategically integrating high-purity, evidence-backed MSM can significantly enhance the competitive positioning of your sports nutrition or joint health line. The final step is to validate the ingredient and the supplier's claims within your own development environment.
To facilitate this with minimal risk, we recommend a hands-on evaluation. Request Your Complimentary Sample & Technical Dossier. This provides physical material for bench-top formulation trials and a complete technical package—including the full CoA, MSDS, and stability data—for review by your quality assurance team. This step is designed to reduce your due diligence burden and provide the concrete evidence needed to make a confident sourcing decision.
Frequently Asked Questions
Share this product
Related Products
Ready to get started?
Contact our team for technical specifications, pricing, and customized solutions.