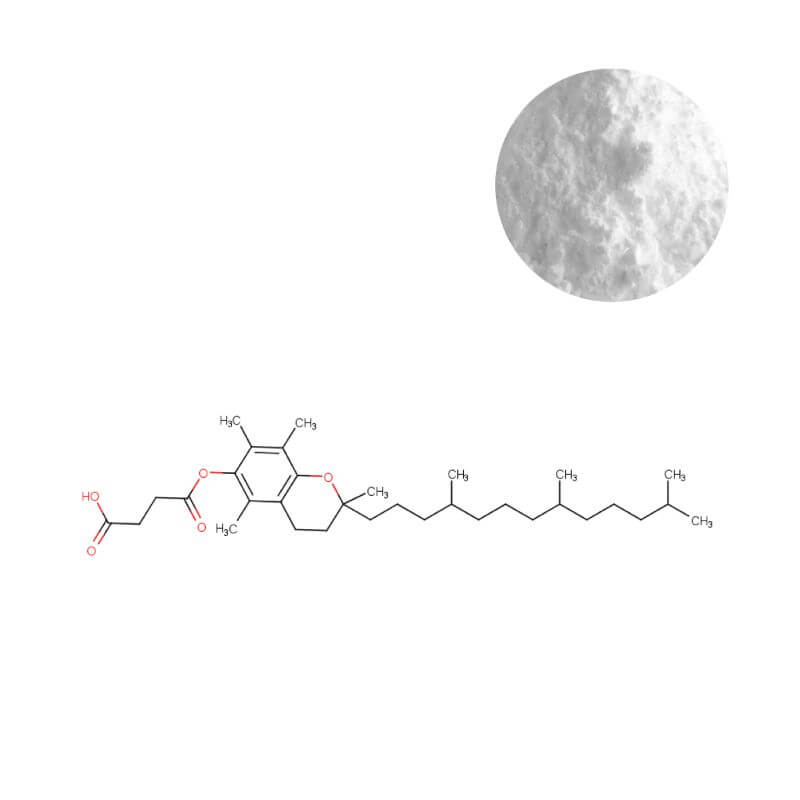
Vitamin E Succinate Powder
| Product Name | Vitamin E Succinate Powder |
| CAS Number | 17407-37-3 |
| Appearance | White to off-white fine powder |
| Activity | 500 IU/g |
| Active Ingredient Content | 50% |
| Packaging | 25 kg/drum |
| MOQ | 1 kg |
Vitamin E Succinate Powder: The Strategic Ingredient for Market-Ready Stability
In nutraceuticals, your ingredient choices directly determine product viability and market longevity. For brands developing solid-dose formats, where oxidation and inconsistent dispersion silently erode potency, the choice of antioxidant is a pivotal business decision. Synthetic Vitamin E Succinate Powder (DL-α-Tocopheryl Succinate, 500 IU/g) transcends basic nutrition. It is an engineered stability solution that addresses manufacturing inefficiencies and shelf-life risk head-on. Its dry-flowable form and superior oxidative resilience are not just technical specs—they are pre-emptive risk mitigation for your product pipeline. This guide provides the framework to evaluate and deploy this ingredient, transforming a raw material into a reliable foundation for brands that compete on consistent quality.
Decoding the Specification Sheet: Why Heavy Metal Limits Trump Purity
For procurement and quality teams, a specification sheet is a risk assessment document. The critical insight? When sourcing bulk Vitamin E Succinate, elemental impurity limits are a more reliable indicator of a supply partner’s rigor than the headline potency figure. A standard enforcing ≤1.0 mg/kg for Lead and ≤0.5 mg/kg for Cadmium (via ICP-MS/USP <232>) signals a commitment to a clean supply chain and advanced purification. This safeguards your brand against escalating regulatory scrutiny in key markets like North America and the EU. The business implication is clear: investing in material with tighter controls substantially reduces the long-tail risk of non-compliance in your finished goods.
Similarly, view the 50% active ingredient content as a deliberate formulation advantage for commercial-scale production. This pre-diluted, carrier-blended standardization is engineered for predictable flow and uniform dispersion in high-speed blending equipment. For a brand manager, this translates to fewer batch failures from content uniformity issues and more efficient production runs. In contrast, a 100% pure powder often introduces handling challenges that increase cost-per-finished-unit through waste and downtime. Therefore, the CoA and residual solvents report (USP <467>) become essential diligence tools. Consistent batch data here is a proxy for supply chain predictability—a non-negotiable for planning your production calendar. Brands requiring a fully natural profile for premium positioning should evaluate d-α-Tocopherol Powder, acknowledging its distinct stability and cost profile.
The Stability Advantage: From Chemical Form to Finished Product Integrity
Justifying the selection of Vitamin E Succinate begins with understanding its molecular stability. The esterification provides a calculated defense against oxidation—a direct driver of product returns and consumer complaints. This intrinsic stability has a tangible financial impact: it extends the viable formulation window for your R&D team and supports stronger, more defensible shelf-life claims. This is a key asset in retail and e-commerce channels where longevity equates to less spoilage and higher sell-through rates.
The practical takeaway for production managers is that this raw material stability must be preserved through informed handling. A critical, often underestimated factor is environmental humidity control during processing. Maintaining relative humidity below 45% at mixing and staging areas is as vital as light-protective packaging in preserving the ≤2.0% loss on drying specification. This proactive measure prevents clumping and moisture-activated degradation, ensuring the ingredient performs as designed in your formula. For applications outside solid dosage, such as liquids or topical blends, understanding the trade-offs is strategic. Vitamin E Acetate Powder offers similar ester stability for different applications, while Natural Mixed Tocopherols Oil provides a full-spectrum antioxidant profile for liquid formulations.
Formulation in Practice: Mastering Dispersion and Synergy in Solid Dosages
The transition from a qualified raw material to a successful finished product hinges on mastering dispersion physics. The most frequent—and costly—failure point is not the ingredient’s purity, but its incomplete integration into the blend, leading to failed potency assays. The ≥95% through 80 mesh particle size specification is a direct response to this industrial challenge. It is engineered to match the bulk density and flow characteristics of common excipients, minimizing segregation and ensuring every tablet or capsule meets its label claim. For a formulator, adhering to the recommended pre-blending protocol with a portion of the filler is not a suggestion; it is a necessary step to secure batch-to-batch consistency and protect your gross margin from waste.
Strategically, this ingredient’s value multiplies when paired in synergistic matrices. Vitamin E functions not in isolation but as part of an antioxidant network. Building this network deliberately enhances your product’s unique selling proposition. Consider these research-backed combinations:
- Sodium Ascorbate Powder: This partnership creates a regenerative redox cycle, allowing antioxidant activity within the formula to be sustained longer—a compelling point of technical differentiation for your marketing.
- Selenium (as selenomethionine): This collaboration supports the body’s endogenous defense systems, enabling a holistic “cellular protection” narrative that resonates in cognitive and longevity health segments.
For powdered drink applications, ensure the entire mix includes anti-caking agents to maintain the free-flowing properties consumers associate with quality.
Mitigating Supply Chain Risk: The Checklist for Strategic Sourcing
In a volatile global landscape, sourcing is synonymous with risk management. Selecting a Vitamin E Succinate supplier is a decision that affects your product’s quality, availability, and cost structure for years. A rigorous evaluation moves beyond price-per-kilo to assess systemic reliability. Use this industry-standard framework for due diligence to uncover potential vulnerabilities before they disrupt your supply:
- Documentation Transparency & Depth: Insist on a full, batch-specific CoA that includes assay, heavy metals, microbiology, and residual solvents. This document is your first-line legal and quality defense.
- Verifiable Quality System Certifications: Confirm that certifications like FSSC 22000 or ISO 22000 are current and relevant to the manufacturing site. This audit trail is your assurance of embedded GMP.
- Supply Chain Traceability and Resilience: Require clear articulation of source security and contingency plans. Proven traceability and dual-sourcing strategies indicate a partner invested in continuity.
- Technical Partnership Capability: Seek partners who offer formulation support or stability data. This signals an investment in your application success, transforming a vendor into an extension of your R&D capability.
Employing this checklist fundamentally shifts the procurement metric from unit price to Total Cost of Ownership (TCO). A partner that excels across these points minimizes hidden costs related to quality investigations, production delays, and inventory obsolescence, delivering superior long-term value to your brand.
Validating the Strategic Fit for Your Brand
Synthetic Vitamin E Succinate Powder represents a convergence of nutritional science and production-grade engineering. It provides a stable, manageable, and efficacious foundation for brands committed to delivering consistent antioxidant performance in sophisticated solid-dose formats. The commercial logic is compelling: it de-risks formulation, strengthens shelf-life claims, and supports clean-label aspirations through stringent impurity controls.
The most effective way to assess its fit for your specific product roadmap is through empirical testing. Request your complimentary sample & technical dossier. This provides not only the physical material for prototyping but also the complete portfolio of technical documentation, allowing your teams to conduct a thorough, evidence-based evaluation and move forward with confidence.
Frequently Asked Questions
Share this product
Related Products
Ready to get started?
Contact our team for technical specifications, pricing, and customized solutions.