5-MTHF vs. Folic Acid: The Strategic Ingredient Choice for Science-Driven Formulators
For formulators, choosing between active 5-MTHF and folic acid is critical for efficacy and positioning. This guide provides a strategic framework to navigate the scientific and commercial trade-offs, ensuring your product delivers on its promise.

Introduction: Beyond Equivalency – A Decision of Consequence
In nutraceutical and food fortification, Vitamin B9 is non-negotiable. Yet, the industry's pivotal choice is no longer about simply "including folate." It's a strategic pivot point between two fundamentally different molecules: the synthetic precursor, folic acid, and the directly bioactive form, L-methylfolate (5-MTHF). Treating them as direct equivalents is a common and costly error. This decision shapes everything from your product's core efficacy narrative and stability profile to its acceptable health claims and target demographic. The key takeaway is that there is no universal "best" choice—only the optimal choice for your specific product goals. Precision in this selection is the hallmark of a sophisticated, scientifically-grounded brand.
Part 1: The Biochemical Imperative – Why Metabolism Dictates Efficacy
The commercial implications of the 5-MTHF versus folic acid debate are rooted in human biochemistry. Understanding these divergent metabolic pathways is essential for predicting real-world product performance and consumer outcomes.
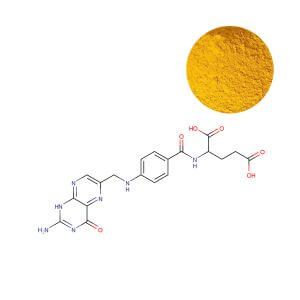
Folic Acid: A Reliable Workhorse with Inherent Limitations
Synthetic folic acid is a stable, oxidized compound requiring complex, multi-step enzymatic conversion in the liver to become bioactive (L-5-MTHF). This pathway is saturable. Our experience suggests that while effective for raising serum folate in the general population, a significant limitation emerges at higher intakes and within specific genetic subgroups. Excess unconverted folic acid circulates as unmetabolized folic acid (UMFA), a subject of ongoing scientific scrutiny regarding its long-term physiological role.
The Commercial Reality: Folic acid's efficacy is contingent upon the consumer's enzymatic efficiency. For the estimated 40-60% of the global population with reduced MTHFR enzyme activity due to common genetic polymorphisms, this conversion is inefficient, rendering folic acid a suboptimal source. This makes it a powerful tool for population-level fortification but a potential weakness in targeted, high-potency, or premium formulations where guaranteed bioavailability is the value proposition.
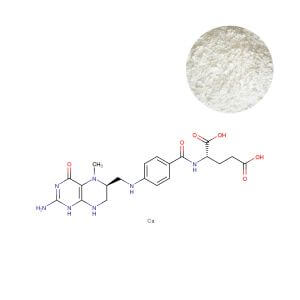
5-MTHF (L-Methylfolate): The Guaranteed Bioactive Endpoint
5-MTHF Calcium is chemically identical to the predominant form of folate circulating in human blood. It bypasses the faulty MTHFR-dependent conversion step entirely, offering passive, direct absorption. One critical factor often overlooked is that this guarantees consistent biological activity regardless of the consumer's genetic makeup.
The Commercial Reality: By formulating with the bioactive form, you eliminate a key variable in product performance. This allows for robust, defensible claims related to efficacy in supporting neurological, cardiovascular, and reproductive health. It positions your product as a premium, science-forward solution, directly appealing to informed consumers and healthcare practitioners who understand the limitations of synthetic folic acid. It transforms folate delivery from a hopeful conversion process into a guaranteed outcome.
Part 2: The Application Framework – Mapping Ingredient to Strategy
The science informs a clear strategic map. Your product's intended market, positioning, and price point will almost automatically point to the correct ingredient choice.
When Folic Acid Is the Strategically Sound Choice
Folic acid remains the undisputed champion for applications where cost-effectiveness, extreme stability, and broad public health mandates are the primary drivers. It is the ingredient of scale.
- Mass-Market Food & Beverage Fortification: Its exceptional thermal and pH stability is unmatched for fortifying staples like flour, pasta, bread, and cereals. The cost-in-use is minimal, making it viable for large-scale public health programs and value-brand products.
- Economical Multivitamins & Baseline Prenatals: For products competing on price and aiming to meet the Recommended Daily Intake (RDI) for a general audience, folic acid is the established, low-risk standard with decades of safety data.
- Compliance with Global Fortification Mandates: Most government-led fortification programs are built around folic acid specifications. Using it ensures straightforward regulatory compliance in these defined categories.
For these core applications, Folic Acid Vitamin B9 Powder is a benchmark ingredient. The real challenge here is not ingredient selection, but securing a reliable, GMP-compliant supply chain that ensures consistent potency for bulk manufacturing.
When 5-MTHF Delivers a Definitive Competitive Edge
Choose 5-MTHF when your product's value proposition hinges on superior, guaranteed absorption and targeted health benefits. This is the choice for differentiation.
- Targeted Premium Supplements: Formulas for cognitive support, mood balance, advanced cardiovascular health, or fertility are fundamentally more credible when they deliver the bioactive form. Marketing can authentically engage with the "methylation" and "MTHFR" conversation prevalent in niche, high-value consumer segments.
- High-Potency Formulas & Practitioner Channels: At doses exceeding 400 mcg DFE, using 5-MTHF circumvents the UMFA debate entirely. This is a critical risk-mitigation strategy for brands sold through healthcare practitioners, where scientific rigor is paramount.
- Innovative Functional Foods & Drinks with Specific Claims: For a premium functional beverage claiming "advanced cellular energy" or "neurological support," 5-MTHF provides a direct, defensible link between ingredient and effect. A more effective approach is to use this ingredient superiority as a key pillar of your product's story.
Scenario: Imagine a brand launching two prenatal lines: a mass-market capsule for broad retail and a premium, direct-to-consumer liquid for fertility-focused consumers. Using folic acid in the first and 5-MTHF in the second perfectly aligns cost structure with value proposition and target audience expectation.
Part 3: Navigating Formulation Realities and Regulatory Complexity
Your strategic choice must be executed with technical precision. Here, the practical differences between the ingredients become operationally critical.
Formulation Stability: From Theory to Practice
While folic acid is famously robust, 5-MTHF demands—and rewards—more sophisticated handling. Modern stabilized salts (like calcium) have made it formulator-friendly, but specific protocols are non-negotiable:
- pH Management: 5-MTHF stability is optimal in slightly acidic to neutral matrices (pH 4-7). Formulating a high-pH beverage? This requires careful buffering or alternative delivery systems.
- Managing Reactive Ingredient Interactions: Direct contact with elemental forms of minerals like iron and copper in pre-mixes can accelerate oxidation. The industry-standard solution is to use microencapsulated or coated mineral forms, creating a physical barrier within the blend.
- Packaging as a Stability Strategy: For 5-MTHF-based supplements, treat packaging as part of the formulation. Blister packs, opaque bottles with oxygen-absorbing desiccants, and nitrogen flushing are not just "nice-to-haves" but essential investments in shelf-life assurance.
The Regulatory Patchwork: Your Market Access Gatekeeper
This is often the decisive commercial factor. Folic acid enjoys near-universal acceptance. The landscape for 5-MTHF is positive but fragmented, requiring proactive verification:
- United States: Specific GRAS-affirmed forms (e.g., (6S)-5-MTHF calcium) are approved for use in supplements and specified food categories.
- European Union: Approved as a Novel Food (EU 2017/1790) for use in supplements, FSMPs, and certain fortified foods with strict maximum levels.
- Global Variability: Status in markets like Canada, Australia, and across Asia varies from approved to case-by-case assessment. For a brand targeting the European market with a fortified snack bar, this means confirming the specific maximum level for the food category is commercially viable before committing to 5-MTHF.
A common industry challenge is navigating this patchwork without delaying time-to-market. Early engagement with a partner who has current, market-specific regulatory intelligence is a key risk-mitigation step.
Part 4: The Strategic Decision Checklist
Translate this analysis into actionable business criteria. Before your next development meeting, ground the discussion in these questions:
- Core Value Proposition: Are we competing on "comprehensive nutrition at value" or "guaranteed, advanced efficacy"?
- Target Consumer Mindset: Are we serving the general, undifferentiated shopper, or a health-engaged consumer actively seeking methylated nutrients and genetic inclusivity?
- Channel & Price Architecture: Is this for mass-market retail with razor-thin margins, or for a premium DTC/practitioner channel where performance justifies price?
- Claim Ambition: Do we need to make claims about "superior absorption," "suitable for MTHFR support," or "direct bioactive form"? If yes, only 5-MTHF supports this.
- Regulatory & Supply Chain Risk: Have we validated the ingredient's status in all target markets and secured a GMP-certified supply chain that ensures batch-to-batch consistency and documentation?
Considering the premium supplement segment is growing at an estimated 8-12% annually, the strategic allocation of 5-MTHF into your innovation pipeline can capture this high-value growth.
Conclusion: From Ingredient Selection to Strategic Formulation
The journey from folic acid to 5-MTHF mirrors the evolution of the nutraceutical industry itself: from generic nutrition to personalized, guaranteed efficacy. Folic acid remains the indispensable public health tool, a testament to its stability and scale. 5-MTHF is the precision instrument for scientific innovation and targeted health solutions.
The ultimate action for product developers is to stop viewing this as a simple ingredient swap and start treating it as a foundational product strategy session. Does your roadmap include a line extension that commands a 30% price premium based on superior science? Does your mass-market product face cost pressures that make folic acid non-negotiable? The answer to these questions dictates your path.
Navigating this complex decision requires more than a data sheet; it demands a partner with applied formulation experience and a clear-eyed view of global regulations. If you are evaluating the strategic role of Vitamin B9 in your portfolio and seek to de-risk the development process with technical and regulatory insights, our team provides targeted formulation support and market-specific guidance to turn this critical choice into your competitive advantage.
Share this article
Found this helpful? Share it with others!
Related Products
Products mentioned in this article
Want to learn more?
Explore our products or contact our team for personalized solutions and expert advice.