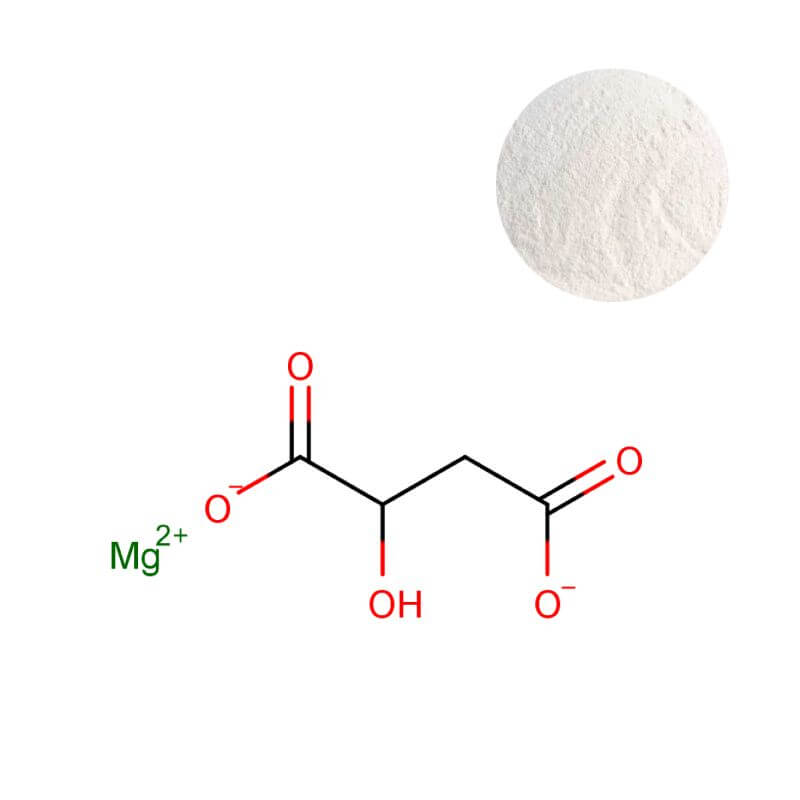
Dimagnesium Malate Powder
| Product Name | Dimagnesium Malate Powder |
| CAS Number | 869-06-7 |
| Appearance | White to off-white powder |
| Purity | 15.5% min. (as Magnesium) |
| Packaging | 25 kg/drum |
| MOQ | 25 kg |
Dimagnesium Malate Powder: Engineered Bioavailability for Targeted Energy & Muscle Support Formulations
In the performance nutrition sector, increasing consumer sophistication means differentiation now depends on a brand's ability to articulate a precise, scientifically-grounded mechanism of action. Simply including "magnesium" on a label is no longer sufficient. The strategic challenge for R&D and procurement teams is to select a mineral form that aligns with a specific health positioning—such as cellular energy or muscular function—while ensuring optimal bioavailability and a clean label. Dimagnesium Malate Powder meets this challenge through its unique biochemical design, which pairs bioavailable magnesium with the Krebs cycle intermediate, malate. Sourced to a specification of ≥15.5% elemental magnesium and characterized by a consistent particle size, this ingredient provides the foundation for targeted, research-backed formulations. The following analysis provides a decision-making framework for evaluating and deploying this sophisticated ingredient to achieve both functional and commercial objectives for bulk formulations.
The Dual-Action Mechanism: Beyond Simple Magnesium Replenishment
Selecting a magnesium form based solely on elemental content is a common oversight. The strategic choice hinges on the complementary physiological role of the anion. Dimagnesium malate is specifically engineered for formulations targeting cellular energy and muscular metabolism. It serves as an excellent source of bioavailable magnesium, an essential mineral cofactor for over 300 enzymatic reactions, most notably those involved in adenosine triphosphate (ATP) synthesis and muscle contraction. The critical differentiator is the malate anion, a key substrate in the mitochondrial Krebs cycle, the primary pathway for cellular energy generation.
This synergy is supported by human clinical data. A randomized, double-blind, placebo-controlled study utilizing a sustained-release formula containing dimagnesium malate demonstrated significant outcomes. After 90 days of supplementation, subjects experienced a 30% increase in red blood cell magnesium levels—a robust marker of magnesium status—concurrent with a 63% improvement in a questionnaire assessing a wide range of magnesium deficiency-related symptoms; 91% reported good tolerance (DOI: 10.1080/07315724.2017.1398686). For a brand manager, this clinical profile provides a clear, defensible narrative distinct from generic mineral supplements. It directly enables claims focused on supporting energy production at the cellular level, a premium positioning compared to magnesium citrate, which is more frequently associated with digestive regularity. Consequently, this ingredient is a strategic fit for products in the sports nutrition, active aging, or holistic energy categories where explaining “how it works” is as important as the functional outcome.
Decoding the CoA: Critical Specifications for Performance and Compliance
A technical specification sheet is not just a quality document; for procurement professionals, it is a risk mitigation tool. When evaluating bulk dimagnesium malate, the elemental magnesium percentage is a basic checkpoint. The true assessment of a supplier’s capability lies in the depth and transparency of the accompanying Certificate of Analysis (CoA). This document must validate parameters that impact performance, stability, and regulatory acceptance.
- Assay and State of Hydration: While the elemental magnesium content (≥15.5%) confirms potency, a low Loss on Drying (LoD) result (e.g., ≤0.5%) is equally critical. This verifies the anhydrous form, ensuring precise, consistent dosing in every batch and eliminating variable water content that could compromise stability in moisture-sensitive formulations.
- Particle Size Distribution: A specification such as "100% through 80 mesh" directly affects manufacturability and consumer experience. Consistent particle size ensures homogeneous blending, prevents segregation in powder mixes, and influences dissolution rate—a key consideration for "fast-acting" product claims.
- Heavy Metals and Microbiology: Adherence to strict global pharmacopeial limits is non-negotiable for brand protection. A comprehensive CoA detailing results for lead, arsenic, cadmium, mercury, and microbiological contaminants provides the documentation necessary for GMP audits and regulatory filings, substantially reducing compliance risk.
From a market access perspective, dimagnesium malate benefits from a formal safety evaluation by the European Food Safety Authority (EFSA) for use as a source of magnesium in food supplements (DOI: 10.2903/j.efsa.2018.5292). For a brand planning a launch in the EU or other markets that recognize EFSA opinions, this pre-existing assessment translates to faster time-to-market and lower regulatory burden compared to a novel ingredient without such a dossier. A seasoned procurement checklist will always include verification of such regulatory status documents from the supplier.
Formulation in Practice: Application, Synergy, and Ingredient Differentiation
The transition from a quality ingredient to a successful finished product depends on practical formulation intelligence. Dimagnesium malate's favorable solubility and relatively neutral taste profile offer formulation flexibility, but its hygroscopic nature requires informed handling. The following table outlines key considerations for major delivery formats, providing a practical guide for R&D teams.
| Format | Key Considerations & Use Level | Processing & Stability Insight |
|---|---|---|
| Powdered Drink Mixes & Stick Packs | Ideal application. Use levels typically target 100-250mg elemental Mg per serving. Excellent for synergistic blends with B-vitamins, electrolytes, and adaptogens. | Hygroscopic. Blending should occur in a controlled environment with humidity below 45% RH to prevent clumping. The fine particle size ensures good dispersion without grittiness. |
| Capsules & Tablets | Suitable for concentrated doses. Its consistent density aids in uniform die-fill. Can be direct-encapsulated or blended. | Compatible with common excipients. Its inherent flow properties can reduce the required amount of lubricants like magnesium stearate, which is used primarily as a processing aid, not a nutrient source. |
| Functional Gummies & Chews | A growing application for great-tasting delivery. Best added post-gelatin cook during the cool-down phase to avoid thermal degradation. | Stable in the final gel matrix at typical pH ranges (3.0-4.2). Formulators should note that its inclusion may slightly reduce the need for additional acidulants to achieve a tart flavor profile. |
For formulators building a comprehensive energy or muscle health matrix, dimagnesium malate pairs effectively with creatine, L-Leucine (a key component of BCAAs), and potassium. A 2019 systematic review confirmed that organic acid salts like malate and citrate generally offer higher bioavailability than oxide forms, but crucially noted that the choice should align with the intended health outcome (DOI: 10.3390/nu11071663). This presents a clear product development strategy: selecting magnesium malate is a deliberate choice for a premium product line where cellular energy metabolism is the central functional and marketing narrative, justifying a potential price premium over more generic mineral blends. The formulation challenge thus shifts from simple nutrient inclusion to optimizing a delivery system that protects and delivers this specific functional synergy.
Strategic Sourcing: A Checklist for Mitigating Supply and Quality Risk
In B2B ingredient sourcing, the lowest unit price often carries the highest total cost of ownership if it introduces quality or supply chain risk. Evaluating a supplier for a specialized ingredient like dimagnesium malate requires a due diligence framework focused on consistency, transparency, and strategic partnership.
- Documentation Depth and Transparency: Does the supplier provide a full, batch-specific CoA with stated analytical methodologies (e.g., USP, ICP-MS)? The absence of this is a major red flag, shifting the burden of quality verification onto the brand.
- Regulatory Support and GMP Status: Can they provide evidence of GMP-compliant manufacturing and relevant regulatory documents (e.g., the EFSA opinion)? This support is critical for brands lacking extensive in-house regulatory resources.
- Consistency and Scalability Proof: Can they demonstrate batch-to-batch consistency over time? Do they have robust upstream supply agreements to secure your long-term production needs?
- Technical Partnership Value: Beyond supply, do they offer application data or formulation support? This depth of service can accelerate development cycles and de-risk scale-up.
Given the scientific consensus that the chemical form dictates bioavailability (DOI: 10.3390/nu11071663), supplier due diligence is directly linked to finished product efficacy. A failure in ingredient consistency is a direct threat to brand equity, as it can lead to variable product performance and consumer dissatisfaction. Therefore, the strategic sourcing decision weighs the value of guaranteed consistency and technical support against the marginal savings of a less-documented source. For a brand, the true cost is measured not just per kilogram, but in the risk of product failure, recall, or lost consumer trust.
Next Steps for Your Product Development
Dimagnesium malate powder offers a validated pathway to create differentiated, science-led products in competitive health categories. The strategic decision to adopt it involves evaluating its unique mechanism, stringent quality specifications, and formulation requirements against your brand's specific positioning and capability. To move from evaluation to integration with confidence, a data-driven approach is essential.
We recommend procuring a comprehensive sample alongside its full technical dossier. This allows your R&D team to assess not just the ingredient's specifications on paper, but its real-world performance in your chosen application matrix. Request Dimagnesium Malate Powder Sample & Technical Dossier to begin a practical evaluation based on complete transparency and hard data.
Frequently Asked Questions
Share this product
Related Products
Ready to get started?
Contact our team for technical specifications, pricing, and customized solutions.